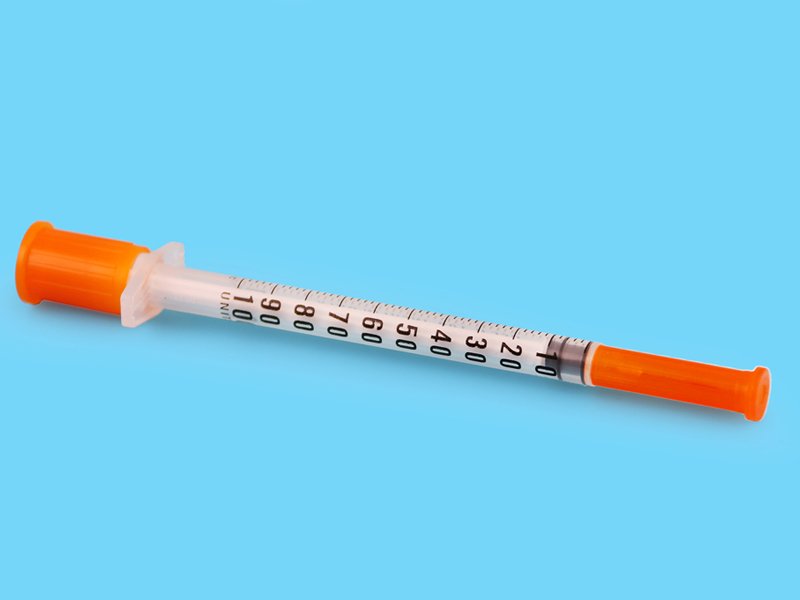
Disposable Insulin Syringes
Categories :
Medical, Syringes
Description :
Disposable Insulin Syringes
Main Material: Medical grade PP,Stainlesssteel or rubber
SIZE:0.3ml U-100/U-40,0.5ml U-100/U-40,1ml U-100/U-40
Intended Use
It is intended for injection of U-100/U-40 insulin into the body.
Characteristic:
- The high transparency of the barrel permits the end-user a strict control on the foreign substance and liquid flow
- The inerasable graduation permits an easy reading, 40 units and 100 units are available
- The medical-grade silicone oil is used as the lubricant for the purpose of getting a smooth plunger movement
- Sterilized by EO gas, non-toxic, non-pyrogenic, single-use only
- Material for barrel and plunger: medical grade PP (polypropylene)
- Material for gasket natural ribber/latex-free
- Fixed needle, no dead space
- Ultrafine needle (27-31G)
- Individual pack: Soft Blister/Polybag
Structure Properties Products
- The integrated design of the needle holder and barrel injection molding vshich ensures the low residue of the liquid andensures the accuracy of the injection of the liquid.Small tip configuration which could reduces the patients pain. Firm adhesion of the ink of scale, friction resistance, not easy to fall off. Excellent sliding performance, convenient for clinical injection operation of medical staff.
- Good usability design: The barrel is transparent and can clearly observe the liquld and bubbles: the plunger has anor-slip structure and is casy to be hold: ant-slip structure of the inner chamber of the back end of the barrel, whichcan prevent the plunger from accidentally slipping out of the barrel: the color of the cap distinguishes insulin strength(U-100 U-40) so that it is easy to identify and use.
| MODEL or TYPE | Carton dimension/Q’TY(pcs) | 40HQ Q’TY(CTN) | 40GQ Q’TY(CTN) | 20GQ Q’TY(CTN) |
| 0.3ml U-100/U-40 | 48.5×39.5×28.5cm/1800pcs | 1260 | 1095 | 520 |
| 0.5ml U-100/U-40 | 48.5×39.5×28.5cm/1800pcs | 1260 | 1095 | 520 |
| 1ml U-100/U-40 | 63.5×36.5×31.5cm/2000pcs | 946 | 816 | 380 |
Notes
Product Conformance
- Conform to ISO 8537and ISO 7864 etc.;
- In compliance with European Medical Device Driective MDD 93/42/EEC:1993( 2007/47/EC)
Quality Assurance
- Manufacturing process is in compliance with ISO 13485 and ISO 9001 Quality System.
Products
Discover Related products
Our products are widely used and provide one-stop solutions